No products in the cart.
Research
Androgenetic Alopecia
Causes, symptoms and treatment options.
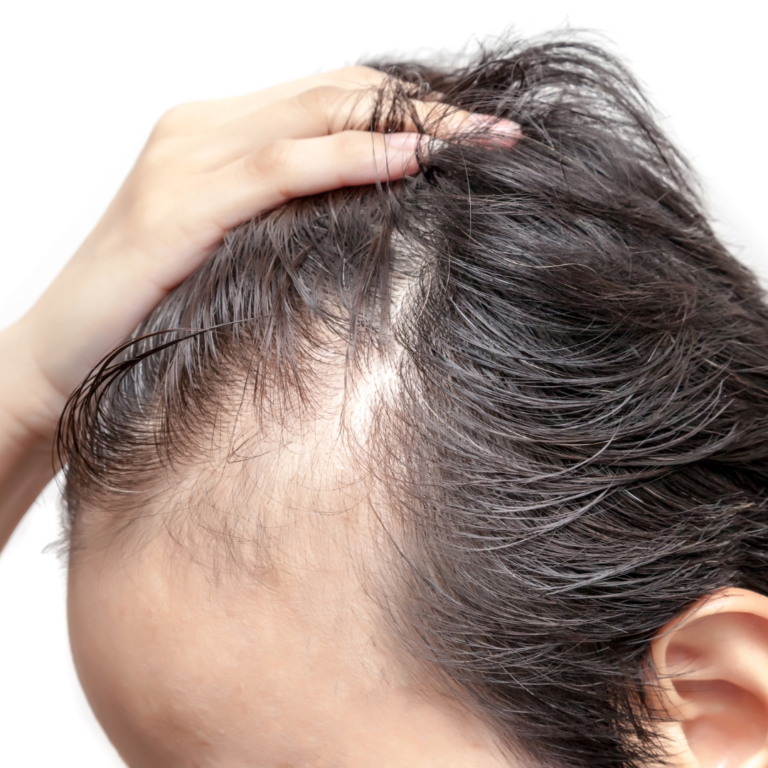
Androgenetic alopecia, also known as pattern baldness, is a hereditary condition characterized by progressive hair loss. It typically follows a specific pattern, such as a receding hairline in men and thinning hair on the crown in women. This alopecia is one of the most common forms of hair loss in both men and women and can have a significant impact on self-esteem and confidence. Androgenetic alopecia is influenced by a combination of genetic and hormonal factors. Genetic predisposition plays a significant role, with certain genes making individuals more susceptible to hair follicle sensitivity to dihydrotestosterone (DHT), a hormone that leads to hair miniaturization and eventual hair loss.
Causes And Symptoms of Alopecia
Common symptoms of androgenetic alopecia include gradual hair thinning, a receding hairline, or a widening parting line. The progression of the condition varies from person to person, and it can lead to significant hair loss over time if left untreated. Diagnosing androgenetic alopecia involves a thorough medical evaluation, including a physical examination, analysis of medical history, and sometimes blood tests to rule out underlying conditions that may contribute to hair loss. Consulting a healthcare professional is essential for an accurate diagnosis.
Treatments options for Androgenetic Alopecia
While managing androgenetic alopecia, individuals can adopt self-care practices and coping strategies. These may include using styling techniques to make hair appear fuller and exploring activities that boost confidence and self-esteem. Various treatment options are available for managing androgenetic alopecia. Topical minoxidil, an over-the-counter medication, can help slow down hair loss and stimulate regrowth. Oral finasteride, a prescription medication, inhibits the production of DHT and can be effective for certain individuals. Hair transplant surgery is another option for restoring hair in areas of significant loss.
Verteporfin
Verteporfin, which is FDA-approved for treating eye disease. They aim to remove the scars. For effective scar treatment, you need to maintain the structure of the epidermis, hair follicles, sebaceous glands, and dermis appendages. The researchers anesthetized surgical wounds on mice and immediately treated the incisions with verteporfin. The results were astonishing. The incision leaves no scars and each site regrows hair follicles. A typical scar has no hair follicles. So how will this affect the hair transplant industry? Read on. Verteporfin works by blocking the Engrailed-1 fibroblast pathway. The Engrailed-1 pathway signals a functional cellular response to the wound, resulting in the development of scar tissue. By blocking the Engrailed-1 pathway, the researchers found that skin wounds no longer leave scars. Wounds are healed by regeneration, and hair follicles, glands, and extracellular matrix are completely regenerated. By inhibiting Engrailed-1 signaling, the wound heals without scarring and with all skin appendages. Now Verteporfin is not capable of significantly treating male pattern baldness. However, combined with surgical hair restoration, it can effectively cure hair loss as we know it.
GT20029
GT20029 is the world’s first topical PROTAC compound to complete Phase 1 clinical trials in China and the United States.
Kintor Pharmaceutical Limited’s GT20029 is safe and well-tolerated. It has shown good pharmacokinetic properties in healthy subjects and subjects with androgenic alopecia (AGA) or acne, based on a phased clinical trial.
Results showed that GT20029 was safe and well tolerated at all doses in all groups. No treatment-related adverse events (TEAEs) related to GT20029 during SAD were reported. The most common TEAEs in the MAD stage are mild and include dry skin, itching, burning, and pain. No serious side effects have been reported. No cases of severe TEAE (grade ≥ 3) and no deaths caused by TEAE have been reported.
Scube3:
SCUBE3 is a potential treatment option for the treatment of male hormonal alopecia.
A signaling molecule called SCUBE3, discovered by researchers at the University of California.
The study, recently published in the journal Developmental Cell, uncovered the exact mechanism by which dermal papilla cells, specialized signal-producing fibroblasts, are found at the bottom of each hair follicle, encouraging new development. Although the important role of dermal papillary cells in regulating hair growth is widely established, the genetic basis of the activating chemicals involved is poorly understood.
For mice and humans to effectively grow hair, cells in the dermis must produce activating chemicals. The skin papilla cells are underactive in people with androgenic alopecia, significantly reducing the trigger chemicals that are usually abundant. This model will help researchers learn more about hair growth regulation.
Pyrilutamide
Pyrilutamide (developmental code name KX-826) is a topical androgen receptor (AR) antagonist being developed as a potential topical treatment for androgenetic alopecia.
The drug innovator is Kintor Pharmaceutical Limited, known for developing and commercializing innovative treatments for RA-related illnesses with unmet medical needs such as male hormone alopecia, acne, and breast and prostate cancer. Its accessibility is almost complete and there are ongoing clinical trials to further prove its effectiveness and safety, which will eventually lead to FDA approval.
Based on clinical trials, 0.5% pyrilutamide applied once daily for female AGA and twice daily for male AGA showed the greatest therapeutic efficacy.
Pyrilutamide is a new topical medication that has the potential to become a first-line drug for hair loss in women and men. Due to its good efficacy and safety profile, based on completed clinical trials, this Kintor drug is a good addition to two existing FDA-approved medications for male hormonal alopecia.